Double Dose of Vaccine For Small Children
Younger children will need two doses of the vaccine against the new pandemic of H1N1 influenza, U.S. officials announced on Monday.
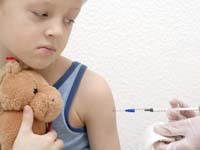
They said tests of Sanofi-Pasteur's (SASY.PA) swine flu vaccine showed children respond to it just as they do to seasonal flu vaccines, with children over 10 needing only a single dose.
Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, said young children will likely need to have their doses 21 days apart. But he said they could receive seasonal flu shots and H1N1 shots on the same day -- something that could ease the logistics of vaccinating children multiple times.
Children aged 10 to 17 mounted an immune response that should protect them from H1N1 within 8 to 10 days.
The U.S. Centers for Disease Control and Prevention said 46 U.S. children have died from swine flu, which appears to have first emerged in Mexico in March and which spread around the world to cause a pandemic in only six weeks.
As soon as the new virus was identified, the U.S. government and companies started making a vaccine against it and testing began in August to ensure it was safe and to determine what dose would be needed.
About 25 companies globally are now making H1N1 vaccine.
The United States had ordered 195 million doses of H1N1 vaccine from five makers -- GlaxoSmithKline (GSK.L), Sanofi, Australia's CSL (CSL.AX), AstraZeneca's (AZN.L) MedImmune unit and Novartis NOVN.AX. With the new order from Sanofi, that would make more than 222 million doses.
This is not enough to cover the U.S. population of 300 million people but the CDC says almost every year influenza vaccines go unused and millions of doses are thrown away.
Most countries ordered H1N1 vaccine with the expectation that two doses would be needed, so the many now have more than anticipated, according to Reuters' report.
Subscribe to Pravda.Ru Telegram channel, Facebook, RSS!




