FDA Engages In Study on Laser Surgery Effects
The Food and Drug Administration intends to study the scope of problems linked to laser eye-correcting surgery, which include blurred vision and dry eyes.
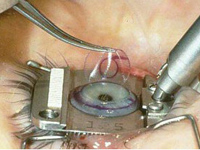
The FDA says it will cooperate with the National Eye Institute and the Department of Defense to detect the percentage of patients who experience negative side effects following surgery.
The first phase of the project is already under way, with plans for an online questionnaire to help patients gauge their quality of life following surgery, according to an FDA statement.
"This study will enhance our understanding of the risks of Lasik and could lead to a reduction in patients who experience adverse effects," said Dr. Jeffrey Shuren, the acting head of FDA's medical device division.
An estimated 6 million Americans have undergone Lasik surgery, which permanently reshapes the cornea, a clear layer covering the eye.
Ophthalmology societies report that about 95 % of patients are satisfied with their new vision.
But a small number of patients have reported permanent damage to their eyes following the surgery, including double vision, dry eye and halos around objects at night.
The FDA agreed to look into the problems in 2008 after years of complaints. The agency said last summer it received 140 reports of Lasik-related problems between 1998 and 2006.
Lasik procedures have fallen off in the past year as consumers cut back on the pricey surgeries, which can cost between $1,500 and $5,000
Also on Thursday, the FDA announced warning letters sent to 17 Lasik surgery centers for inadequate adverse event reporting procedures. Regulators periodically send letters to facilities that don't follow federal guidelines for reporting patient complaints.
More inspections of Lasik centers are planned in coming months, according to the FDA release.
The Associated Press contributed to the report.
Subscribe to Pravda.Ru Telegram channel, Facebook, RSS!


