H1N1 Vaccine to Suffice For Everyone
Test results of its H1N1 vaccine confirm that children under 10 may need two shots to be fully protected.
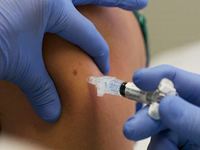
The U.S. Centers for Disease Control and Prevention said the news is not surprising, since this age group needs two doses of regular seasonal flu vaccine for full immunity to develop.
The new Sanofi results back up what government tests are showing, said Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases. For younger children, the protection from one shot is "modest but not sufficient to allow for one dose to do the trick," he said.
Sanofi is the only company licensed in the United States to make vaccine for children as young as 6 months old. The company tested two strengths of the vaccine, given as two shots 21 days apart. The vaccine was tested in 474 children ages 6 months through 9 years old.
Another option for people without medical problems is FluMist, a nasal spray vaccine. It is approved for healthy people 2 to 49 years old. The nasal spray accounts for most of the vaccine available now, although shots are starting to make their way to states. Pregnant women and young children are among the groups most urged to seek the vaccine as soon as it is available.
The Associated Press contributed to the report.
Subscribe to Pravda.Ru Telegram channel, Facebook, RSS!





