US FDA approves German blood plasma substitute Voluven
By Margarita Snegireva. U.S. FDA approved German drug Voluven. It is an intravenous solution that prevents and treats a dangerous loss of blood volume, a condition that sometimes occurs during and after surgery, the federal agency said on Friday.
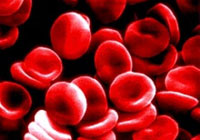
Significant blood losses can cause a rapid drop in the volume of red blood cells and plasma circulating through the body. This can lead to shock, which is potentially fatal.
Blood volume expanders are commonly administered to quickly restore some of the lost volume so that remaining red blood cells can continue to deliver needed oxygen to the body's tissues.
As a new blood volume expander manufactured by Fresenius Kabi in Germany , Voluven contains a synthetic starch that does not dissolve in water. It is made by linking individual starch molecules together and combining them with a salt solution, similar to the salt concentration typically found in blood. Voluven expands the volume of blood plasma and thus draws fluid into small blood vessels.
Hydroxyethyl starch is a nonionic starch derivative. It is one of the most frequently used blood plasma substitutes. It is also used in oil drilling.
Side-effects: Anaphylactoid reactions: hypersensitivity, mild influenza-like symptoms, bradycardia, tachycardia, bronchospasm and non-cardiogenic pulmonary edema.
Decrease in hematocrit and disturbances in coagulation. May be associated with covering the use of anabolic steroids/EPO for endurance athletes
Subscribe to Pravda.Ru Telegram channel, Facebook, RSS!





