America approves generic versions of insomnia drug Ambien
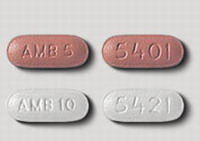
The first generic versions of the insomnia drug Ambien got U.S. government approval Monday.
The Food and Drug Administration said it approved versions of the immediate-release tablets made by 13 drug companies for the short-term treatment of insomnia. A patent held by Paris-based Sanofi-Aventis on the drug, also called zolpidem tartrate, expired Saturday.
The approvals of generic versions of the drug come a month after the FDA asked makers of it and similar sleep aids to beef up warnings about their potential risks. The risks of the sedative-hypnotic drugs include severe allergic reactions and complex sleep-related behaviors, like sleep-driving.
The FDA said Ambien was the No. 13 brand-name drug by sales last year.
Subscribe to Pravda.Ru Telegram channel, Facebook, RSS!