FDA Reveals Counterfeit Alli
The Food and Drug Administration warned consumers Monday about counterfeit and potentially harmful capsules of GlaxoSmithKline's over-the-counter weight loss drug Alli.
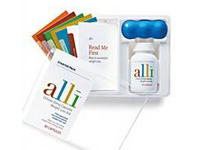
The FDA said counterfeit versions of the 60-milligram capsules in the 120-count refill kit contain the controlled substance sibutramine. The actual product should contain orlistat.
Sibutramine is a drug that certain patients should not use, and it should not be used without physician oversight, the agency said. Also, it can interact in a harmful way with other medications.
The FDA said consumers began reporting suspected counterfeit Alli to GlaxoSmithKline in early December 2009. The company determined the counterfeit product has been sold on the Internet and there is no evidence it has been sold through other channels, such as in retail stores.
The counterfeit Alli looks similar to the real version, with a few differences, including an outer cardboard packaging missing a "Lot" code. Also, the counterfeit version has an expiration date that includes the month, day, and year, while the real version of the drug only includes the month and year. Also, the counterfeit drug packaging is in a plastic bottle that has a slightly taller and wider cap with coarser ribbing than the genuine product.
Other differences include a plain foil inner safety seal under the plastic cap without any printed words, while the authentic product seal is printed with "SEALED for YOUR PROTECTION". The counterfeit capsules are larger with a white powder, instead of small white pellets.
The MSN Money has contributed to the report.
Subscribe to Pravda.Ru Telegram channel, Facebook, RSS!


